

| Tracks 1-16 | ||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you review what we know about bendamustine and
NHL?
DR LOVE: Would you review what we know about bendamustine and
NHL?
![]() DR CHESON: In two trials for patients with rituximab-refractory disease,
bendamustine alone was active, with overall response rates higher than 70
percent (Friedberg 2008; Kahl 2007). Rummel evaluated the combination of
rituximab/bendamustine in patients with a variety of histologies of previously treated indolent lymphoma or mantle-cell lymphoma. The overall response rate
was 90 percent, with a complete remission rate of 60 percent (Rummel 2005).
DR CHESON: In two trials for patients with rituximab-refractory disease,
bendamustine alone was active, with overall response rates higher than 70
percent (Friedberg 2008; Kahl 2007). Rummel evaluated the combination of
rituximab/bendamustine in patients with a variety of histologies of previously treated indolent lymphoma or mantle-cell lymphoma. The overall response rate
was 90 percent, with a complete remission rate of 60 percent (Rummel 2005).
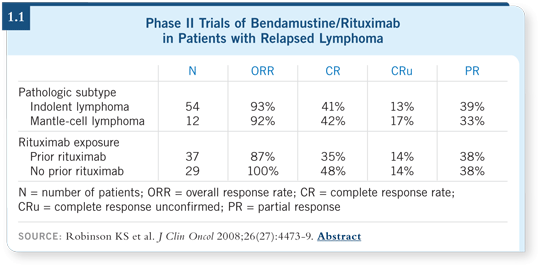
We were able to reproduce these findings with rituximab/bendamustine in patients with indolent histologies, particularly follicular lymphoma or mantlecell lymphoma. Surprisingly, the response rates were identical in the two populations — approximately 92 to 93 percent. These patients had relapsed/refractory disease, although patients with disease that was refractory to rituximab, defined by a less than partial response or a response lasting less than six months, were excluded (Robinson 2008; [1.1]).
These data suggest we have a new option — now that bendamustine has been approved — for patients with non-Hodgkin lymphoma that has failed prior therapies, particularly those with mantle-cell lymphoma, which is an incurable disease without many active therapies.
Track 3
![]() DR LOVE: What are the side effects and toxicities of bendamustine?
DR LOVE: What are the side effects and toxicities of bendamustine?
![]() DR CHESON: Primarily myelosuppression — neutropenia and thrombocytopenia.
The frequency with which these occur depends on the trial and the
disease. In one of our trials, the most common reason for patients coming
off the study was Grade III/IV thrombocytopenia, but that occurred in only
about 10 percent of the patients.
DR CHESON: Primarily myelosuppression — neutropenia and thrombocytopenia.
The frequency with which these occur depends on the trial and the
disease. In one of our trials, the most common reason for patients coming
off the study was Grade III/IV thrombocytopenia, but that occurred in only
about 10 percent of the patients.
Nausea and vomiting can occur but are unpredictable, so we recommend that all patients receive prophylactic antiemetics. An infusion reaction — associated with fevers, chills and muscle aches — has been reported in a small number of patients. In a couple of patients, the serum creatinine increased. This reaction subsides if you discontinue the drug or administer corticosteroids.
Whether the risk of secondary malignancies is associated with bendamustine is not clear.
Tracks 6-7
![]() DR LOVE: Would you discuss the use of lenalidomide in B-cell malignancies?
DR LOVE: Would you discuss the use of lenalidomide in B-cell malignancies?
![]() DR CHESON: Lenalidomide is a second-generation immunomodulatory
drug that has been approved for the treatment of multiple myeloma and
myelodysplastic syndrome (MDS) in patients with the 5q deletion. It has
also been evaluated in patients with indolent (Witzig 2007) and aggressive
(Czuczman 2008) non-Hodgkin lymphoma, for which it has response rates of
about 30 percent, depending on the histology.
DR CHESON: Lenalidomide is a second-generation immunomodulatory
drug that has been approved for the treatment of multiple myeloma and
myelodysplastic syndrome (MDS) in patients with the 5q deletion. It has
also been evaluated in patients with indolent (Witzig 2007) and aggressive
(Czuczman 2008) non-Hodgkin lymphoma, for which it has response rates of
about 30 percent, depending on the histology.
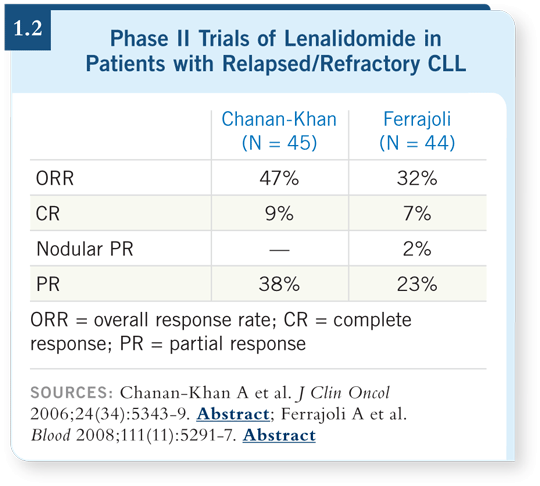
Lenalidomide is also an interesting agent in chronic lymphocytic leukemia (CLL). Two studies using different doses and schedules have demonstrated activity in this setting. Chanan-Khan demonstrated a response rate of approximately 50 percent among patients with relapsed/refractory disease (Chanan-Khan 2006; [1.2]). Ferrajoli from MD Anderson achieved responses in around 35 percent of patients with relapsed/refractory disease (Ferrajoli 2008; [1.2]). We don’t know whether the difference in response rates was due to patient selection, dose or schedule, but lenalidomide is active. Lenalidomide also appears to be active in patients with CLL who have adverse cytogenetics, such as the 11q abnormality and the 17p deletion (Ferrajoli 2007).
You have to be cautious with lenalidomide in patients with CLL. It has the usual side effects of myelosuppression, but two additional adverse effects are of particular concern. The first is a tumor flare reaction. Patients receive the agent for a couple of weeks, and suddenly their nodes increase markedly in size and become painful. The white blood cell count is also elevated. A few days or a week or two later, however, the nodes shrink, the pain goes away, the white count comes down and the patient is well and may even be in remission.
The second potentially serious adverse effect is tumor lysis syndrome, which appears to be dose independent. It has been reported at all doses, including doses as low as 2.5 milligrams per day. It can be life threatening or fatal (Moutouh-de Parseval 2007). The risk of tumor lysis syndrome appears to be especially high for patients with CLL, and we are most concerned about those patients who have increased tumor bulk.
Tracks 13-14
![]() DR LOVE: What are some common questions you receive from oncologists
with regard to patients who have follicular lymphoma?
DR LOVE: What are some common questions you receive from oncologists
with regard to patients who have follicular lymphoma?
![]() DR CHESON: The big question is, “What is the best initial therapy for these
patients?” Patients can receive R-CHOP, R-CVP or single-agent rituximab.
Right now we don’t know whether initial therapy will make any difference
10 or 15 years down the line, because we have many effective salvage therapies.
I practice a risk-adapted approach.
DR CHESON: The big question is, “What is the best initial therapy for these
patients?” Patients can receive R-CHOP, R-CVP or single-agent rituximab.
Right now we don’t know whether initial therapy will make any difference
10 or 15 years down the line, because we have many effective salvage therapies.
I practice a risk-adapted approach.
If patients have bulky disease or need immediate therapy because they are symptomatic or organs are compromised, I lean toward R-CHOP. If the patient has progressive disease that’s not big but probably needs to be treated, I may use R-CVP. I rarely use single-agent rituximab. It has a reasonable response rate, but the responses tend to be short-lived.
Other questions include, “What is the role of maintenance rituximab in follicular lymphoma?” and, “What is the optimal schedule for maintenance rituximab?”
Five different strategies have been published for maintenance rituximab: four doses every six months for two years, one dose every two months times four, one dose every three months for two years, one dose every three months until disease progression and a regimen based on serum rituximab levels. We don’t know which is the best approach. We don’t know which patients, if any, benefit from maintenance rituximab.
We also don’t know whether maintenance rituximab benefits patients who have been treated with rituximab/chemotherapy. This question is being addressed by the PRIMA trial, in which patients were treated with a regimen selected by their institution — R-CHOP, R-CVP or R-FCM — and then randomly assigned to maintenance rituximab or not. The data from this study are maturing. If they show a meaningful benefit, it will certainly affect practice.
Maintenance rituximab is not innocuous. It is expensive and requires patients to come in to the office for an injection. More neutropenic infections and hospitalizations are reported with maintenance rituximab than without it. An increased risk of progressive multifocal leukoencephalopathy has also been reported in a small number of patients with lymphoma who received rituximab.
Data in the relapse setting suggest a survival benefit with maintenance rituximab. In a study of CHOP versus R-CHOP and maintenance rituximab versus no maintenance for patients with follicular lymphoma, a progression-free and overall survival benefit was found with maintenance rituximab (van Oers 2006; [1.3]).

| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Bruce D Cheson, MD
- Select publications
Hagop M Kantarjian, MD
- Select publications
Sagar Lonial, MD
- Select publications