

| Tracks 1-20 | ||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 1-2
![]() DR LOVE: What are some of the key clinical research issues related to the
use of imatinib in patients with chronic myelogenous leukemia (CML)?
DR LOVE: What are some of the key clinical research issues related to the
use of imatinib in patients with chronic myelogenous leukemia (CML)?
![]() DR KANTARJIAN: Current questions include whether we can discontinue
a patient’s therapy with imatinib after a certain period. We’re working on immune stimulation of the host with either vaccines or peginterferon. Otherwise,
patients will receive imatinib for a lifetime, as people receive medications
for hypertension or diabetes.
DR KANTARJIAN: Current questions include whether we can discontinue
a patient’s therapy with imatinib after a certain period. We’re working on immune stimulation of the host with either vaccines or peginterferon. Otherwise,
patients will receive imatinib for a lifetime, as people receive medications
for hypertension or diabetes.
Also significant is the issue of pregnancy in women receiving imatinib. Most women who become pregnant but stop imatinib deliver normal babies. A recent publication, however, reported three children with a syndrome of skeletal, kidney and eye malformations (Pye 2008). So we cannot confirm that women who are receiving imatinib will have safe pregnancies.
Another question involves resistance to imatinib, which occurs at a rate of approximately four percent per year. Several new-generation tyrosine kinase inhibitors are more potent than imatinib. Some of them are pure BCR-ABL inhibitors similar to imatinib. One of them, nilotinib, is FDA approved and has shown good results in patients for whom imatinib has failed. Among such patients, approximately 50 percent reachieve a complete cytogenetic response, and two-year overall survival is approximately 90 percent (Kantarjian 2008).
A second FDA-approved drug, dasatinib, produces similar results to nilotinib, with a complete cytogenetic response rate of approximately 60 percent and an estimated two-year overall survival of approximately 90 percent (Mauro 2008).
Still another question is whether any of these new-generation tyrosine kinase inhibitors can be moved up to front-line therapy. My assessment is that the results with imatinib are so good that it will be difficult to beat — it will take large, randomized trials with a follow-up of five to seven years. So I believe imatinib is well established as front-line therapy.
![]() DR LOVE: What are some of the important clinical issues in caring for patients
who receive imatinib for long periods?
DR LOVE: What are some of the important clinical issues in caring for patients
who receive imatinib for long periods?
![]() DR KANTARJIAN: One of the most important issues is compliance. Approximately
70 percent of the patients are reported to have some form of noncompliance
(Halpern 2007). It doesn’t mean they stop taking imatinib completely,
but they do miss days of the medication. If we have a patient who shows
resistance to imatinib, the first possibility to consider is noncompliance. A
test to measure plasma imatinib levels is available to determine whether the
resistance is due to noncompliance or other factors, such as the development of
mutations.
DR KANTARJIAN: One of the most important issues is compliance. Approximately
70 percent of the patients are reported to have some form of noncompliance
(Halpern 2007). It doesn’t mean they stop taking imatinib completely,
but they do miss days of the medication. If we have a patient who shows
resistance to imatinib, the first possibility to consider is noncompliance. A
test to measure plasma imatinib levels is available to determine whether the
resistance is due to noncompliance or other factors, such as the development of
mutations.
A second important issue is intolerance. Perhaps five percent of patients are completely intolerant. Most patients demonstrate this intolerance up front with skin rashes, liver function abnormalities or fluid retention.
Track 5
![]() DR LOVE: What are some of the recent important research developments
in the treatment of CLL?
DR LOVE: What are some of the recent important research developments
in the treatment of CLL?
![]() DR KANTARJIAN: I have seen much progress since the discovery of the activity
of fludarabine and the value of adding rituximab to fludarabine. Front-line
treatment for patients with CLL who require therapy is usually a combination of
fludarabine/rituximab or fludarabine/cyclophosphamide and rituximab.
DR KANTARJIAN: I have seen much progress since the discovery of the activity
of fludarabine and the value of adding rituximab to fludarabine. Front-line
treatment for patients with CLL who require therapy is usually a combination of
fludarabine/rituximab or fludarabine/cyclophosphamide and rituximab.
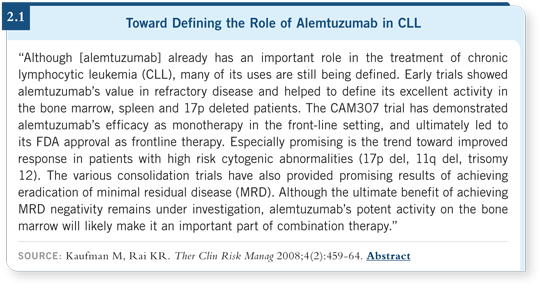
Two other drugs have shown a great deal of promise in the setting of refractory disease. Alemtuzumab is approved for the treatment of refractory disease, and lenalidomide has shown activity across a range of disorders, such as multiple myeloma, MDS with the 5q abnormality, CLL and some lymphomas.
So the current question is, can we improve on the durability of responses? If a patient with CLL receives FCR therapy, we know the remissions will last for a median of six to eight years. Can we use alemtuzumab or lenalidomide as treatment for minimal residual disease rather than in the setting of failure of front-line therapy (2.1)?
Bendamustine, an old drug with the properties of alkylating agents and adenosine nucleoside analogs, was known to be active by investigators in East Germany about 30 years ago. Bendamustine emerged as one of the most powerful drugs in lymphoid malignancies. A study comparing bendamustine to chlorambucil as front-line therapy for CLL demonstrated its superiority and led to the approval of this indication for bendamustine (Knauf 2007).
We have moved away from chlorambucil in the United States. The approval for bendamustine is somewhat awkward because it is for front-line therapy, but US oncologists universally use fludarabine/rituximab-based therapy before considering bendamustine.
Track 18
![]() DR LOVE: Let’s talk about recent progress in the treatment of MDS
DR LOVE: Let’s talk about recent progress in the treatment of MDS
![]() DR KANTARJIAN: The first important building block was the discovery of the
activity of the hypomethylating agents, azacitidine and decitabine. An international
study comparing azacitidine to a conventional care regimen — low-dose
ara-C, intensive chemotherapy or best supportive care — demonstrated a
distinct survival advantage with azacitidine.
DR KANTARJIAN: The first important building block was the discovery of the
activity of the hypomethylating agents, azacitidine and decitabine. An international
study comparing azacitidine to a conventional care regimen — low-dose
ara-C, intensive chemotherapy or best supportive care — demonstrated a
distinct survival advantage with azacitidine.
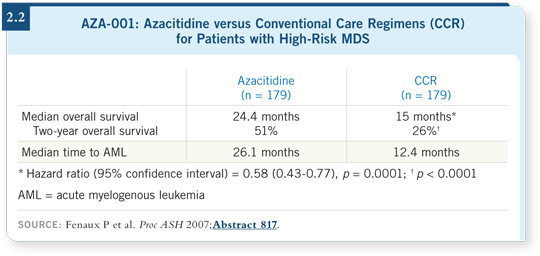
This is the first agent, outside of the setting of allogeneic transplantation, that changes the natural course of MDS. The median overall survival was approximately 25 months with azacitidine compared to 15 months with the standard treatment, and the two-year overall survival almost doubled from 26 to 51 percent (List 2008; Fenaux 2007; [2.2]).
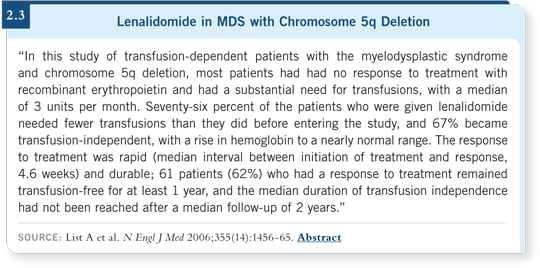
Among patients with lower-risk MDS and a chromosome 5q deletion who were transfusion dependent, lenalidomide has shown a transfusion-independence rate of 66 percent, a complete cytogenetic response rate of about 45 percent and a median duration of response in terms of transfusion independence of approximately 27 months (List 2006; [2.3]).
Tracks 19-20
![]() DR LOVE: Would you discuss your clinical strategy for patients wth MDS?
DR LOVE: Would you discuss your clinical strategy for patients wth MDS?
![]() DR KANTARJIAN: Once the diagnosis is confirmed, we usually observe
patients or, if indications for treatment are present, such as significant anemia,
we use growth factors — erythropoietin with or without G-CSF. Transfusions
are used as needed. We check every patient with MDS for chromosomal
abnormalities for several reasons.
DR KANTARJIAN: Once the diagnosis is confirmed, we usually observe
patients or, if indications for treatment are present, such as significant anemia,
we use growth factors — erythropoietin with or without G-CSF. Transfusions
are used as needed. We check every patient with MDS for chromosomal
abnormalities for several reasons.
The first reason is prognostication. The chromosomal abnormalities are part of the International Prognostic Scoring System. Patients with a chromosome seven abnormality or more than three abnormalities have an average survival of less than one year. You must intervene rapidly for those patients. The second reason to conduct the chromosomal studies is to identify the patients with 5q deletions, who will benefit from lenalidomide (List 2006; [2.3]).
For elderly patients who do not respond to growth factors and require transfusions, if the blasts in the bone marrow are in the range of seven percent or less, I would try some form of immunotherapy, such as cyclosporine, steroids or antithymocyte globulin (ATG), before proceeding with hypomethylating agents.
If patients experience disease progression on these strategies or if they present with higher-risk MDS, then the first choice should be a hypomethylating agent, such as azacitidine because it has demonstrated a survival advantage (List 2008; Fenaux 2007; [2.2]).
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Bruce D Cheson, MD
- Select publications
Hagop M Kantarjian, MD
- Select publications
Sagar Lonial, MD
- Select publications