

| Tracks 1-19 | ||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 3
![]() DR LOVE: What is the role of dose escalation of imatinib for patients with
CML?
DR LOVE: What is the role of dose escalation of imatinib for patients with
CML?
![]() DR O’BRIEN: In a recently published trial, patients failing imatinib at 400 or
600 milligrams were randomly assigned to either imatinib at 800 milligrams
or dasatinib.
DR O’BRIEN: In a recently published trial, patients failing imatinib at 400 or
600 milligrams were randomly assigned to either imatinib at 800 milligrams
or dasatinib.
The data were analyzed according to which dose of imatinib the patient had failed (Kantarjian 2007). Patients who failed while they were receiving imatinib at 600 milligrams were better off switching to dasatinib. For those who had failed while on imatinib at 400 milligrams, the response rates between the two arms were similar, and the improvement in progression-free survival with dasatinib was of borderline significance (Kantarjian 2007).
This is why the NCCN guidelines consider dose escalation of imatinib as an option (NCCN 2008). However, if a patient has never had a cytogenetic response to imatinib, it’s better to switch therapy than to increase the dose.
Track 10
![]() DR LOVE: Would you discuss the six-year follow-up data from the
IRIS trial, evaluating imatinib in the treatment of chronic-phase CML
(Hochhaus 2007)?
DR LOVE: Would you discuss the six-year follow-up data from the
IRIS trial, evaluating imatinib in the treatment of chronic-phase CML
(Hochhaus 2007)?
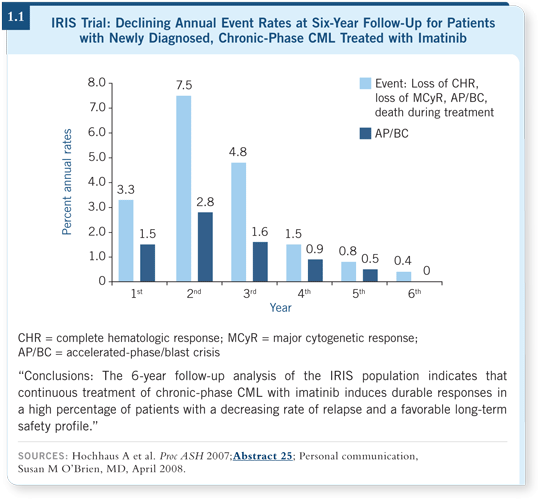
![]() DR O’BRIEN: The failure rate continues to be low, showing imatinib to be
excellent in the front-line setting (1.1). One of the most interesting findings is
that the number of events per year is declining. In fact, during the sixth year,
no patients developed accelerated phase or blast crisis.
DR O’BRIEN: The failure rate continues to be low, showing imatinib to be
excellent in the front-line setting (1.1). One of the most interesting findings is
that the number of events per year is declining. In fact, during the sixth year,
no patients developed accelerated phase or blast crisis.
To some, these data suggest that early on, a clone of imatinib-resistant cells may develop in some patients that is too small to detect with standard techniques. When imatinib eradicates the sensitive clone, the resistant clone emerges and the patients leave the study and experience an event within a year or two. However, patients without a resistant clone have nothing to cause failure — so the failure rate is decreasing.
Track 13
![]() DR LOVE: Let’s talk about CLL. Are we at a point at which we can use a
chromosomal abnormality, such as a 17p deletion, to select therapy?
DR LOVE: Let’s talk about CLL. Are we at a point at which we can use a
chromosomal abnormality, such as a 17p deletion, to select therapy?
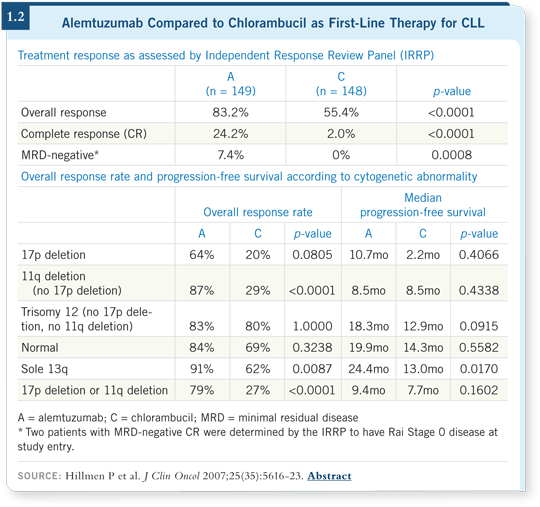
![]() DR O’BRIEN: The simple answer is no. However, we know that patients with
17p deletions don’t respond well to fludarabine-based therapy (Byrd 2006), our
mainstay of treatment. Data show that those patients do respond to alemtuzumab.
In the trial comparing it to chlorambucil as first-line therapy for CLL,
alemtuzumab was better in all groups based on cytogenetic abnormalities
(Hillmen 2007; [1.2]).
DR O’BRIEN: The simple answer is no. However, we know that patients with
17p deletions don’t respond well to fludarabine-based therapy (Byrd 2006), our
mainstay of treatment. Data show that those patients do respond to alemtuzumab.
In the trial comparing it to chlorambucil as first-line therapy for CLL,
alemtuzumab was better in all groups based on cytogenetic abnormalities
(Hillmen 2007; [1.2]).
So patients with 17p deletions fared better on alemtuzumab. Still, their median progression-free survival was 10 months (Hillmen 2007; [1.2]). Alemtuzumab by itself is not the magic bullet for patients with 17p deletions. Steroids also work in these patients, and the British are conducting a Phase II trial combining alemtuzumab with steroids.
Track 14
![]() DR LOVE: Would you discuss some of the ongoing trials with alemtuzumab
for the treatment of CLL?
DR LOVE: Would you discuss some of the ongoing trials with alemtuzumab
for the treatment of CLL?
![]() DR O’BRIEN: We are conducting a study combining alemtuzumab with
fludarabine, cyclophosphamide and rituximab (FCR) for patients at high
risk (2005-0269). We are also conducting a trial using alemtuzumab to treat
minimal residual disease, for which I believe it is particularly effective (2003-0834). Alemtuzumab is not great at treating bulky adenopathy, but it’s excellent
at clearing bone marrow disease.
DR O’BRIEN: We are conducting a study combining alemtuzumab with
fludarabine, cyclophosphamide and rituximab (FCR) for patients at high
risk (2005-0269). We are also conducting a trial using alemtuzumab to treat
minimal residual disease, for which I believe it is particularly effective (2003-0834). Alemtuzumab is not great at treating bulky adenopathy, but it’s excellent
at clearing bone marrow disease.
Emerging data show that we need a certain period of time — probably three to six months — between treatment with fludarabine and consolidation with alemtuzumab to allow recovery of the immune system (Hainsworth 2008). Patients with a reasonable response to first-line therapy do not experience disease progression that quickly, so we have time to wait, repeat the bone marrow biopsy and then use alemtuzumab if needed to eradicate residual disease. In a German randomized trial, this approach was shown to have a major impact on progression-free survival (Wendtner 2004).
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Susan M O’Brien, MD
- Select publications
Robert Z Orlowski, MD, PhD
- Select publications
David P Steensma, MD
- Select publications
David G Maloney, MD, PhD
- Select publications
Hematologic Oncology Update:
A CME Audio Series and Activity