

| Tracks 1-17 | ||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 2-3
![]() DR LOVE: Can you discuss the ECOG trial evaluating lenalidomide
combined with high- and low-dose dexamethasone?
DR LOVE: Can you discuss the ECOG trial evaluating lenalidomide
combined with high- and low-dose dexamethasone?
![]() DR ORLOWSKI: ECOG-E4A03 randomly assigned patients with newly
diagnosed myeloma to lenalidomide with high-dose dexamethasone or
lenalidomide with low-dose dexamethasone. The overall response rate was
about 12 percent lower with low-dose dexamethasone compared to high-dose
dexamethasone, but overall survival was better with low-dose dexamethasone
(Rajkumar 2007). Less intensive therapy, which patients can tolerate better and
benefit from a better overall survival rate, represents an advance in the field.
DR ORLOWSKI: ECOG-E4A03 randomly assigned patients with newly
diagnosed myeloma to lenalidomide with high-dose dexamethasone or
lenalidomide with low-dose dexamethasone. The overall response rate was
about 12 percent lower with low-dose dexamethasone compared to high-dose
dexamethasone, but overall survival was better with low-dose dexamethasone
(Rajkumar 2007). Less intensive therapy, which patients can tolerate better and
benefit from a better overall survival rate, represents an advance in the field.
Track 5
![]() DR LOVE: Can you describe the key first-line induction studies with
bortezomib-based regimens reported at ASH 2007?
DR LOVE: Can you describe the key first-line induction studies with
bortezomib-based regimens reported at ASH 2007?
![]() DR ORLOWSKI: In an important study from the Italian Myeloma Group,
patients with newly diagnosed multiple myeloma were randomly assigned to
thalidomide/dexamethasone with or without bortezomib (VTD or TD) prior
to ASCT (Cavo 2007). This study was designed to administer only three
cycles of three-week induction therapy before patients went on to ASCT
— a reduction in the amount of therapy patients receive prior to transplant,
which is always positive. The patients who received VTD had a higher overall
response rate and better response quality than those receiving TD. Interestingly,
VTD was associated with a better complete and overall response
rate than TD in patients with deletions of chromosome 13 or translocations
between 4 and 14 compared to those without the high-risk features.
DR ORLOWSKI: In an important study from the Italian Myeloma Group,
patients with newly diagnosed multiple myeloma were randomly assigned to
thalidomide/dexamethasone with or without bortezomib (VTD or TD) prior
to ASCT (Cavo 2007). This study was designed to administer only three
cycles of three-week induction therapy before patients went on to ASCT
— a reduction in the amount of therapy patients receive prior to transplant,
which is always positive. The patients who received VTD had a higher overall
response rate and better response quality than those receiving TD. Interestingly,
VTD was associated with a better complete and overall response
rate than TD in patients with deletions of chromosome 13 or translocations
between 4 and 14 compared to those without the high-risk features.
The overall toxicity profile of the two regimens was comparable, with a little more neuropathy associated with VTD but more thromboembolic complications with TD. In general, when bortezomib is incorporated into a regimen, fewer thromboembolic complications occur. We don’t know why this occurs, but it’s a welcome development.
Tracks 7-8
![]() DR LOVE: Can you discuss recent studies of first-line therapy for patients
who are not candidates for transplantation?
DR LOVE: Can you discuss recent studies of first-line therapy for patients
who are not candidates for transplantation?
![]() DR ORLOWSKI: A study from France evaluated melphalan/prednisone (MP)
or MP with thalidomide (MP-T) for patients with newly diagnosed myeloma
who were more than 75 years old and were not considered by most of us
as candidates for transplantation. The patients who received MP-T had a
significant improvement in overall response rate, response quality and time
to progression. Overall survival was improved by 18 months among patients
treated with MP-T (Hulin 2007).
DR ORLOWSKI: A study from France evaluated melphalan/prednisone (MP)
or MP with thalidomide (MP-T) for patients with newly diagnosed myeloma
who were more than 75 years old and were not considered by most of us
as candidates for transplantation. The patients who received MP-T had a
significant improvement in overall response rate, response quality and time
to progression. Overall survival was improved by 18 months among patients
treated with MP-T (Hulin 2007).
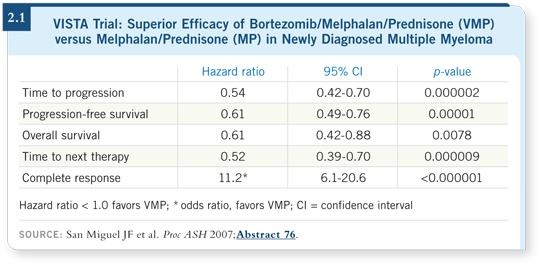
A second trial — VISTA — evaluated MP versus bortezomib with MP (VMP). The patients who received VMP had a superior overall response rate compared to those treated with MP, and adverse cytogenetic effects did not have an impact on overall response or durability of response. The complete response rate was five percent with MP compared to 35 percent with VMP (San Miguel 2007; [2.1]).
For older patients, two good options are now available: MP with bortezomib or MP with thalidomide. These are probably the two best standard treatments for patients who may not be transplant candidates.
Tracks 9,12
![]() DR LOVE: How do you decide between IMiD-based and bortezomib-based
regimens?
DR LOVE: How do you decide between IMiD-based and bortezomib-based
regimens?
![]() DR ORLOWSKI: I believe that patients with adverse cytogenetic features or
those with moderate to high-stage disease according to the International
Staging System should receive a bortezomib-containing regimen. We also
know that bortezomib is safe, effective and doesn’t require dose reductions for
patients with renal failure, which occurs in a substantial proportion of patients
with multiple myeloma. For patients with good-risk cytogenetics, the best
approach is to present both IMiD-based and bortezomib-based options and
to obtain input from the patient. However, I would still argue that the higher
complete response rates with bortezomib-based regimens are worth considering
strongly.
DR ORLOWSKI: I believe that patients with adverse cytogenetic features or
those with moderate to high-stage disease according to the International
Staging System should receive a bortezomib-containing regimen. We also
know that bortezomib is safe, effective and doesn’t require dose reductions for
patients with renal failure, which occurs in a substantial proportion of patients
with multiple myeloma. For patients with good-risk cytogenetics, the best
approach is to present both IMiD-based and bortezomib-based options and
to obtain input from the patient. However, I would still argue that the higher
complete response rates with bortezomib-based regimens are worth considering
strongly.
![]() DR LOVE: What do we know about combining an IMiD and bortezomib?
DR LOVE: What do we know about combining an IMiD and bortezomib?
![]() DR ORLOWSKI: Paul Richardson made a great presentation at ASH of a
Phase I/II study evaluating bortezomib with lenalidomide and dexamethasone
for patients with relapsed or refractory multiple myeloma. They were
able to identify a tolerable dosage, which was safe and had an overall response
rate of more than 90 percent (Richardson 2007). In the future, bortezomib/lenalidomide and dexamethasone may prove to be an optimal regimen for all
patients. Being able to achieve response rates close to 100 percent with shorter
durations of therapy is quite encouraging.
DR ORLOWSKI: Paul Richardson made a great presentation at ASH of a
Phase I/II study evaluating bortezomib with lenalidomide and dexamethasone
for patients with relapsed or refractory multiple myeloma. They were
able to identify a tolerable dosage, which was safe and had an overall response
rate of more than 90 percent (Richardson 2007). In the future, bortezomib/lenalidomide and dexamethasone may prove to be an optimal regimen for all
patients. Being able to achieve response rates close to 100 percent with shorter
durations of therapy is quite encouraging.
Track 15
![]() DR LOVE: Would you comment on your Phase III study of liposomal
doxorubicin and bortezomib in relapsed or refractory multiple myeloma?
DR LOVE: Would you comment on your Phase III study of liposomal
doxorubicin and bortezomib in relapsed or refractory multiple myeloma?
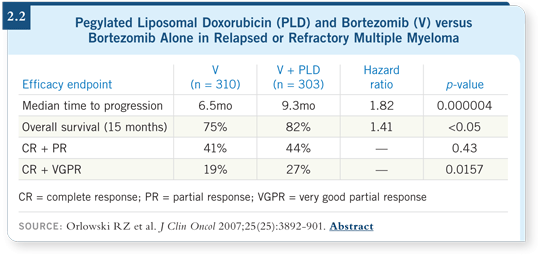
![]() DR ORLOWSKI: This was the first trial demonstrating that an anthracycline in
combination with bortezomib had a better overall response rate and quality of
response than bortezomib alone. The very good partial response plus complete
response rate went from about 20 percent to 30 percent, which was a 50 percent
improvement (Orlowski 2007; [2.2]). We also saw a trend toward better overall
survival, which I believe will continue as the data mature. The data show that
the benefits of bortezomib and pegylated liposomal doxorubicin were maintained
regardless of the patients’ age, prior transplant or exposure to thalidomide.
DR ORLOWSKI: This was the first trial demonstrating that an anthracycline in
combination with bortezomib had a better overall response rate and quality of
response than bortezomib alone. The very good partial response plus complete
response rate went from about 20 percent to 30 percent, which was a 50 percent
improvement (Orlowski 2007; [2.2]). We also saw a trend toward better overall
survival, which I believe will continue as the data mature. The data show that
the benefits of bortezomib and pegylated liposomal doxorubicin were maintained
regardless of the patients’ age, prior transplant or exposure to thalidomide.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Susan M O’Brien, MD
- Select publications
Robert Z Orlowski, MD, PhD
- Select publications
David P Steensma, MD
- Select publications
David G Maloney, MD, PhD
- Select publications
Hematologic Oncology Update:
A CME Audio Series and Activity