

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Where are we right now in terms of therapy for myelodysplastic
syndrome (MDS)?
DR LOVE: Where are we right now in terms of therapy for myelodysplastic
syndrome (MDS)?
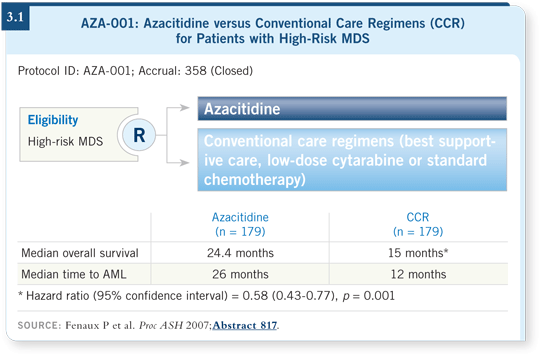
![]() DR STEENSMA: The biggest news, which came from the 2007 ASH meeting,
was the presentation of data from a study comparing azacitidine to the conventional
care regimens of best supportive care, low-dose cytarabine or standard
chemotherapy. Azacitidine was shown to improve overall survival by about nine
months and delay transformation to leukemia. It was also well tolerated (Fenaux
2007; [3.1]). Patients received an average of nine cycles of therapy, so they were
able to receive the drug for a longer period than we’ve seen in the past.
DR STEENSMA: The biggest news, which came from the 2007 ASH meeting,
was the presentation of data from a study comparing azacitidine to the conventional
care regimens of best supportive care, low-dose cytarabine or standard
chemotherapy. Azacitidine was shown to improve overall survival by about nine
months and delay transformation to leukemia. It was also well tolerated (Fenaux
2007; [3.1]). Patients received an average of nine cycles of therapy, so they were
able to receive the drug for a longer period than we’ve seen in the past.
Another drug in the same class, decitabine, is approved for myelodysplasia. A multicenter study with a five-day outpatient regimen of decitabine — which is more convenient than the regimen on the package labeling — demonstrated a 32 percent complete response rate. That is better than what we have seen before azacitidine and decitabine were available (Steensma 2007).
![]() DR LOVE: How would you compare the available data for azacitidine and
decitabine?
DR LOVE: How would you compare the available data for azacitidine and
decitabine?
![]() DR STEENSMA: We have survival data for azacitidine (Fenaux 2007; [3.1])
but not yet for decitabine. EORTC-06011 is an ongoing study of decitabine
in which survival is the endpoint. We’re likely to hear those results later this
year or perhaps in early 2009. The trial is taking place in Europe, with a study
design similar to the azacitidine trial. Azacitidine and decitabine have never
been compared directly, so we have to extrapolate by comparing studies side
by side.
DR STEENSMA: We have survival data for azacitidine (Fenaux 2007; [3.1])
but not yet for decitabine. EORTC-06011 is an ongoing study of decitabine
in which survival is the endpoint. We’re likely to hear those results later this
year or perhaps in early 2009. The trial is taking place in Europe, with a study
design similar to the azacitidine trial. Azacitidine and decitabine have never
been compared directly, so we have to extrapolate by comparing studies side
by side.
Track 2
![]() DR LOVE: What current clinical research for MDS do you expect to have
the greatest impact on clinical practice during the next three to five years?
DR LOVE: What current clinical research for MDS do you expect to have
the greatest impact on clinical practice during the next three to five years?
![]() DR STEENSMA: One interesting area is combining azacitidine or decitabine
with other classes of drugs. People are most excited about the combinations
with the histone deacetylase (HDAC) inhibitors. One of those, vorinostat,
is already approved for cutaneous T-cell lymphoma. Several others are being
evaluated specifically in MDS. ECOG-E1905 is comparing azacitidine to
azacitidine with an HDAC inhibitor called MS-275. A Phase II multicenter trial is evaluating another HDAC inhibitor, belinostat. If it shows efficacy as a
single agent, we may have a good rationale to combine it.
DR STEENSMA: One interesting area is combining azacitidine or decitabine
with other classes of drugs. People are most excited about the combinations
with the histone deacetylase (HDAC) inhibitors. One of those, vorinostat,
is already approved for cutaneous T-cell lymphoma. Several others are being
evaluated specifically in MDS. ECOG-E1905 is comparing azacitidine to
azacitidine with an HDAC inhibitor called MS-275. A Phase II multicenter trial is evaluating another HDAC inhibitor, belinostat. If it shows efficacy as a
single agent, we may have a good rationale to combine it.
Combining azacitidine and decitabine with the HDAC inhibitors is attractive because the side-effect profiles are different. With the HDAC inhibitors, cytopenias don’t seem to be an issue as much as QT-interval prolongation and fatigue (Byrd 2005). Perhaps we could use the agents together and not find much overlap of the adverse events.
![]() DR LOVE: What do we know about the side effects and toxicities of azacitidine
and decitabine?
DR LOVE: What do we know about the side effects and toxicities of azacitidine
and decitabine?
![]() DR STEENSMA: With azacitidine, the biggest issue has been cytopenias.
Neutropenia is manageable for some patients, but it lands others in the hospital
with febrile neutropenia. In the Phase II multicenter study of decitabine,
17 percent of the patients had febrile neutropenia (Steensma 2007). That was
not as high as with some of the leukemia induction regimens, but it’s not
negligible either.
DR STEENSMA: With azacitidine, the biggest issue has been cytopenias.
Neutropenia is manageable for some patients, but it lands others in the hospital
with febrile neutropenia. In the Phase II multicenter study of decitabine,
17 percent of the patients had febrile neutropenia (Steensma 2007). That was
not as high as with some of the leukemia induction regimens, but it’s not
negligible either.
The other adverse events associated with azacitidine and decitabine, which are similar, are mild: Gastrointestinal toxicities and rash.
Track 5
![]() DR LOVE: Would you discuss your clinical approach to the treatment of
patients with MDS?
DR LOVE: Would you discuss your clinical approach to the treatment of
patients with MDS?
![]() DR STEENSMA: I start by risk stratifying. Is the patient at high or low risk of
progression to leukemia and death? If the patient is at low risk, you have time
to try different approaches, such as growth factors. If the patient is at high risk,
then the question is whether he or she is a transplant candidate. I find that
assessment difficult.
DR STEENSMA: I start by risk stratifying. Is the patient at high or low risk of
progression to leukemia and death? If the patient is at low risk, you have time
to try different approaches, such as growth factors. If the patient is at high risk,
then the question is whether he or she is a transplant candidate. I find that
assessment difficult.
The transplant centers are accepting sicker and older patients now. I don’t automatically rule out someone who is 63 or 64 years old. I send them to the transplant physician to hear the specialist’s opinion.
If the transplant physician recommends it, then for higher-risk disease, transplant is the treatment of choice. We may need to prepare the patient for the transplant and decrease the blast count with azacitidine, but transplant is the definitive therapy.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Susan M O’Brien, MD
- Select publications
Robert Z Orlowski, MD, PhD
- Select publications
David P Steensma, MD
- Select publications
David G Maloney, MD, PhD
- Select publications
Hematologic Oncology Update:
A CME Audio Series and Activity