

| Tracks 1-12 | ||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Would you discuss the presentation of the azacitidine trial
(AZA-001) at ASH 2007 by Fenaux?
DR LOVE: Would you discuss the presentation of the azacitidine trial
(AZA-001) at ASH 2007 by Fenaux?
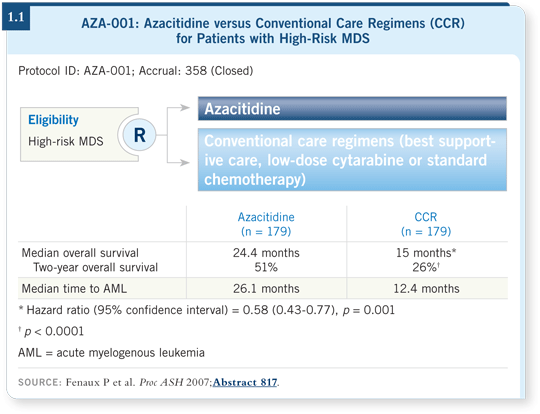
![]() DR GORE: This seminal study was conducted primarily in Europe, and the
participating doctors selected one of three conventional care regimens (Fenaux
2007; [1.1]). The trial was set up this way because in Europe a great deal of
variability existed in what was considered the standard treatment. For instance,
in France they used a lot of low-dose cytarabine, and in Italy almost everyone
received supportive care.
DR GORE: This seminal study was conducted primarily in Europe, and the
participating doctors selected one of three conventional care regimens (Fenaux
2007; [1.1]). The trial was set up this way because in Europe a great deal of
variability existed in what was considered the standard treatment. For instance,
in France they used a lot of low-dose cytarabine, and in Italy almost everyone
received supportive care.
The three choices were (1) best supportive care — including growth factors and transfusions, (2) low-dose cytarabine or (3) standard chemotherapy. Once the physician selected one of those three, the patient was randomly assigned to either that choice or azacitidine at the FDA-approved dose and schedule. Patients received azacitidine or the conventional care regimen until disease progression (Fenaux 2007; [1.1]).
This trial enrolled patients with high-risk myelodysplastic syndrome (MDS), and the median overall survival was improved by approximately nine months among those treated with azacitidine. More impressively, overall survival at two years in this high-risk group was approximately doubled, from 26 to 51 percent, which is extraordinary and clinically meaningful (Fenaux 2007; [1.1]).
Track 2
![]() DR LOVE: How do you choose between azacitidine and decitabine in
clinical practice?
DR LOVE: How do you choose between azacitidine and decitabine in
clinical practice?
![]() DR GORE: I have conducted a lot of research on these compounds, and it’s my
belief that biologically they’re similar. If they were administered in equally
toxic or equally effective dosing schedules, they would probably be quite
similar. However, they have been developed clinically in highly different
schedules.
DR GORE: I have conducted a lot of research on these compounds, and it’s my
belief that biologically they’re similar. If they were administered in equally
toxic or equally effective dosing schedules, they would probably be quite
similar. However, they have been developed clinically in highly different
schedules.
The FDA-approved schedule for decitabine, a three-day intravenous inpatient schedule administered every six weeks, is probably a more toxic regimen than the azacitidine regimen in terms of hematologic toxicity. Two survival trials used that decitabine regimen. One, published by Kantarjian in Cancer a couple of years ago, showed no survival benefit compared to observation (Kantarjian 2006). I believe that the trial showed no survival benefit because the median number of cycles that were administered was only two to three.
For both of these agents, to obtain hematologic benefit you need to use at least four to six cycles. All of the azacitidine trials have, for the most part, enabled patients who didn’t achieve a complete remission to receive ongoing therapy. However, none of the decitabine trials have used more than eight cycles of therapy. So the duration of response always appears greater in the azacitidine trials, which I believe suggests that maintenance therapy is important.
The EORTC ran a similar trial comparing supportive care to the FDA-approved regimen of decitabine for patients with high-risk MDS. Although it was only reported as a press release, they didn’t meet their target endpoint, which is to say that decitabine did not improve overall survival (Eisai 2008). Hence we have two drugs that biologically are similar but have different doses and schedules. One doubles survival at two years, and one doesn’t. To me, it’s incumbent on the physician to prescribe azacitidine.
Alternative dosing schedules for decitabine, which are better tolerated — particularly those developed at MD Anderson — have been studied only in Phase II trials (Kantarjian 2007), and they haven’t been studied for their impact on survival. Again, I believe that if you’re interested in improving a patient’s survival, outside of a clinical trial, the data suggest using azacitidine at the FDA-approved dose and schedule.
Tracks 5, 7
![]() DR LOVE: Could you discuss entinostat and how it’s being evaluated?
DR LOVE: Could you discuss entinostat and how it’s being evaluated?
![]() DR GORE: Entinostat (MS-275) is one of the second-generation histone
deacetylase (HDAC) inhibitors. It’s been studied in AML in a Phase I study,
and it clearly has some monotherapy activity (Gojo 2007). We chose to
combine it with azacitidine in a Phase I study, which we presented at ASH.
Although one should not extrapolate from a Phase I study, responses were
promising (Gore 2006). We’re currently conducting a Phase II randomized
trial (ECOG-E1905; [1.2]) comparing the combination of azacitidine and
entinostat to azacitidine alone in AML and MDS.
DR GORE: Entinostat (MS-275) is one of the second-generation histone
deacetylase (HDAC) inhibitors. It’s been studied in AML in a Phase I study,
and it clearly has some monotherapy activity (Gojo 2007). We chose to
combine it with azacitidine in a Phase I study, which we presented at ASH.
Although one should not extrapolate from a Phase I study, responses were
promising (Gore 2006). We’re currently conducting a Phase II randomized
trial (ECOG-E1905; [1.2]) comparing the combination of azacitidine and
entinostat to azacitidine alone in AML and MDS.
![]() DR LOVE: What’s been observed with the HDAC inhibitors in terms of side
effects and toxicities?
DR LOVE: What’s been observed with the HDAC inhibitors in terms of side
effects and toxicities?
![]() DR GORE: The HDAC inhibitors tend to induce significant asthenia if the
dose is pushed. It is the limiting factor. Even with the FDA-approved dose and
schedule of vorinostat for cutaneous T-cell lymphomas, patients are extremely
fatigued. Many of them have some nausea, but fatigue is the biggest problem.
HDAC inhibitors have also been associated with cardiac QT interval issues.
The HDAC inhibitor that’s drawn the most attention is romidepsin, also
known as depsipeptide. For a while it was thought to be particularly cardiotoxic,
but that’s probably not true (Klimek 2008; Byrd 2005). Certainly, EKG
changes must be monitored, which seems to be a class effect.
DR GORE: The HDAC inhibitors tend to induce significant asthenia if the
dose is pushed. It is the limiting factor. Even with the FDA-approved dose and
schedule of vorinostat for cutaneous T-cell lymphomas, patients are extremely
fatigued. Many of them have some nausea, but fatigue is the biggest problem.
HDAC inhibitors have also been associated with cardiac QT interval issues.
The HDAC inhibitor that’s drawn the most attention is romidepsin, also
known as depsipeptide. For a while it was thought to be particularly cardiotoxic,
but that’s probably not true (Klimek 2008; Byrd 2005). Certainly, EKG
changes must be monitored, which seems to be a class effect.
The side-effect profile of our combination of azacitidine and entinostat does not appear to be significantly different from that of azacitidine alone at the doses of entinostat we’re administering, which are low (Gore 2006). Of course, it will require the randomized Phase II trial results to compare the adverse events of the two arms.

| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Steven D Gore, MD
- Select publications
Michael J Keating, MB, BS
- Select publications
William I Bensinger, MD
- Select publications
Fredrick B Hagemeister, MD
- Select publications