

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 4
![]() DR LOVE: Would you discuss the evolution of treatment options for
patients with chronic lymphocytic leukemia?
DR LOVE: Would you discuss the evolution of treatment options for
patients with chronic lymphocytic leukemia?
![]() DR KEATING: We’ve gone from single-agent chlorambucil — with a response
rate of approximately 50 to 60 percent and a three to five percent complete
remission rate — to fludarabine — with a higher overall response rate and a
20 to 25 percent complete remission rate — to the combinations of fludarabine/cyclophosphamide (FC), fludarabine/rituximab (FR), fludarabine/cyclophosphamide/rituximab (FCR) or pentostatin/cyclophosphamide/rituximab.
All of these combinations have evolved during the past eight to nine years of
clinical trials.
DR KEATING: We’ve gone from single-agent chlorambucil — with a response
rate of approximately 50 to 60 percent and a three to five percent complete
remission rate — to fludarabine — with a higher overall response rate and a
20 to 25 percent complete remission rate — to the combinations of fludarabine/cyclophosphamide (FC), fludarabine/rituximab (FR), fludarabine/cyclophosphamide/rituximab (FCR) or pentostatin/cyclophosphamide/rituximab.
All of these combinations have evolved during the past eight to nine years of
clinical trials.
The observation made by the CALGB that fludarabine/rituximab is superior to fludarabine alone (Byrd 2005) has been confirmed by a study performed in Europe (GCLLSG-CLL-8), in which the German CLL Study Group compared FC to FCR. FCR has now been established as having a higher complete remission rate and a longer progression-free survival rate (Roche 2008). It is now widely accepted that chemoimmunotherapy with rituximab is superior to chemotherapy alone.
Track 5
![]() DR LOVE: What about alemtuzumab and bendamustine?
DR LOVE: What about alemtuzumab and bendamustine?
![]() DR KEATING: Chlorambucil has been compared to alemtuzumab. The
study demonstrated higher complete and overall response rates and a longer
progression-free survival rate with alemtuzumab compared to chlorambucil.
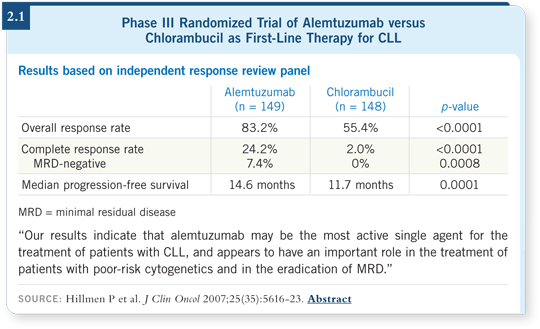
However, no overall survival advantage has been noted (Hillmen 2007; [2.1]).
DR KEATING: Chlorambucil has been compared to alemtuzumab. The
study demonstrated higher complete and overall response rates and a longer
progression-free survival rate with alemtuzumab compared to chlorambucil.
However, no overall survival advantage has been noted (Hillmen 2007; [2.1]).
A presentation at ASH compared bendamustine, which is an alkylating agent, to chlorambucil. That study demonstrated a significantly higher complete response rate, overall response rate and progression-free survival rate with bendamustine (Knauf 2007).
Track 6
![]() DR LOVE: Would you discuss how alemtuzumab and bendamustine fit
into the overall treatment algorithm for CLL?
DR LOVE: Would you discuss how alemtuzumab and bendamustine fit
into the overall treatment algorithm for CLL?
![]() DR KEATING: In the front-line setting, alemtuzumab may be used for patients
who don’t want to receive chemotherapy, which may be predominantly the
elderly patient population. Whether doctors in the United States will be
interested in doing that is questionable. Alemtuzumab is extremely potent in
clearing the peripheral blood. In five to six days, all the leukemic cells are
gone. It’s fairly effective in the marrow and spleen but not effective in bulky
lymph nodes.
DR KEATING: In the front-line setting, alemtuzumab may be used for patients
who don’t want to receive chemotherapy, which may be predominantly the
elderly patient population. Whether doctors in the United States will be
interested in doing that is questionable. Alemtuzumab is extremely potent in
clearing the peripheral blood. In five to six days, all the leukemic cells are
gone. It’s fairly effective in the marrow and spleen but not effective in bulky
lymph nodes.
Bendamustine is generating a lot of interest because of a suggestion that it is a better alkylating agent. In lymphoma, bendamustine seems to be as good or perhaps slightly better than CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) in comparisons of CHOP with rituximab to bendamustine with rituximab (Rummel 2007). Bendamustine is administered for two days, so it’s relatively convenient. It may cause cumulative suppression of the marrow, but we need more data on this and more US experience before we can draw as many conclusions as we wish.
Track 8
![]() DR LOVE: Would you discuss the combination of alemtuzumab with
rituximab?
DR LOVE: Would you discuss the combination of alemtuzumab with
rituximab?
![]() DR KEATING: The combination was put together predominantly by our
group at MD Anderson on the basis that rituximab performed well in terms
of shrinking lymph nodes but did not perform well in the bone marrow, and
alemtuzumab performed well in the marrow and not in the lymph nodes. Our
thinking was that because we had two antigen targets, CD20 and CD52, it
was possible that the two antibodies together would have a greater effect.
DR KEATING: The combination was put together predominantly by our
group at MD Anderson on the basis that rituximab performed well in terms
of shrinking lymph nodes but did not perform well in the bone marrow, and
alemtuzumab performed well in the marrow and not in the lymph nodes. Our
thinking was that because we had two antigen targets, CD20 and CD52, it
was possible that the two antibodies together would have a greater effect.
We conducted a four-week program with four conventional doses of rituximab and twice-a-week alemtuzumab. You might expect a 20 to 25 percent partial response rate. However, with four weeks of treatment, approximately 40 percent of the patients experienced partial remission and five to 10 percent experienced complete remission (Faderl 2003).
My thought, without direct proof, is that the drugs are greater than additive and are probably synergistic. An advantage is that the whole truncated course is finished in four weeks. Patients have rapid responses, and we don’t see any toxicity with the combination beyond what you might see with alemtuzumab or rituximab alone.
Track 10
![]() DR LOVE: Let’s talk about the management of chronic myelogenous
leukemia.
DR LOVE: Let’s talk about the management of chronic myelogenous
leukemia.
![]() DR KEATING: The complete cytogenetic and molecular response rates are so
good that imatinib is obviously the treatment of choice. A dose of 400 milligrams
is great, but would 600 milligrams or 800 milligrams be better? The
responses appear to be quicker but not necessarily better on a long-term basis,
and more toxicity appears with the higher doses (Cortes 2008).
DR KEATING: The complete cytogenetic and molecular response rates are so
good that imatinib is obviously the treatment of choice. A dose of 400 milligrams
is great, but would 600 milligrams or 800 milligrams be better? The
responses appear to be quicker but not necessarily better on a long-term basis,
and more toxicity appears with the higher doses (Cortes 2008).
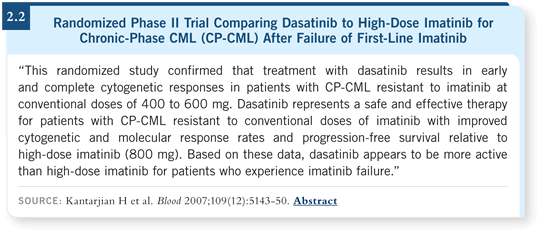
Before the new tyrosine kinase inhibitors dasatinib and nilotinib were available, patients who were not faring well would receive imatinib dose escalations, and some responded (Kantarjian 2003). Now a higher dose of imatinib has been compared to dasatinib. The time to treatment failure was superior with dasatinib compared to the higher dose of imatinib (Kantarjian 2007; [2.2]). I believe that most people are simply switching to a different tyrosine kinase inhibitor rather than increasing the dose of imatinib.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Steven D Gore, MD
- Select publications
Michael J Keating, MB, BS
- Select publications
William I Bensinger, MD
- Select publications
Fredrick B Hagemeister, MD
- Select publications