

| Tracks 1-10 | ||||||||||||||||||||||
|
Select Excerpts from the Interview
Track 2
![]() DR LOVE: Would you discuss the data on clofarabine as treatment for AML and MDS?
DR LOVE: Would you discuss the data on clofarabine as treatment for AML and MDS?
![]() DR GARCIA-MANERO: We have published data from Phase I and Phase II
studies in AML with Dr Faderl from MD Anderson as lead author in a
number of papers in Blood (Faderl 2005, 2006, 2008a). Response rates with
clofarabine or clofarabine/ara-C are similar to those for patients with the same
characteristics treated with idarubicin/ara-C (IA)-type chemotherapy.
DR GARCIA-MANERO: We have published data from Phase I and Phase II
studies in AML with Dr Faderl from MD Anderson as lead author in a
number of papers in Blood (Faderl 2005, 2006, 2008a). Response rates with
clofarabine or clofarabine/ara-C are similar to those for patients with the same
characteristics treated with idarubicin/ara-C (IA)-type chemotherapy.
Studies of clofarabine in older patients are ongoing, and data from a key study are now being analyzed. It’s possible that lower doses — 10 mg/m2 to 30 mg/m2 — may be well tolerated, with mortality rates of approximately five or 10 percent, which is lower than with the standard approaches of the past. We have to wait and see whether that translates into a meaningful improvement in survival.
The question is, can you extrapolate data in AML to MDS? We are involved in a number of studies, both at MD Anderson and in cooperative studies, evaluating lower doses and oral schedules of clofarabine for patients with MDS.
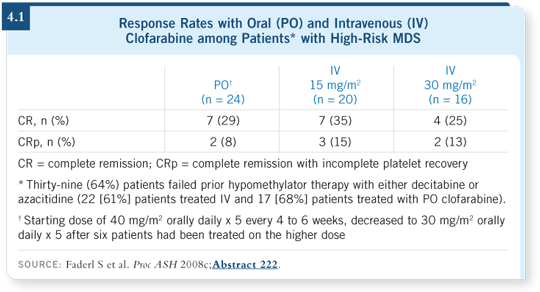
The data are exciting and have been presented at the last two ASH annual meetings (Faderl 2007, 2008c). The response rate is approximately 40 to 50 percent. I have seen a strong signal that clofarabine may allow rescue of some patients for whom 5-azacitidine or decitabine fails (Faderl 2008c; [4.1]). I believe that this may be a major breakthrough in helping control the disease for patients who have not been helped by hypomethylating agents.
Tracks 3-4
![]() DR LOVE: Cost and reimbursement issues aside, how would you use
clofarabine as treatment for AML and MDS?
DR LOVE: Cost and reimbursement issues aside, how would you use
clofarabine as treatment for AML and MDS?
![]() DR GARCIA-MANERO: Clofarabine would be my first choice for a patient with
MDS for whom either 5-azacitidine or decitabine has failed. In AML, clofarabine
would also be a consideration. However, the reality is that we have more
experience with other approaches with which cost issues are not so relevant,
such as fludarabine-containing regimens.
DR GARCIA-MANERO: Clofarabine would be my first choice for a patient with
MDS for whom either 5-azacitidine or decitabine has failed. In AML, clofarabine
would also be a consideration. However, the reality is that we have more
experience with other approaches with which cost issues are not so relevant,
such as fludarabine-containing regimens.
The study of clofarabine versus clofarabine/ara-C as front-line therapy for patients age 60 or older with AML or high-risk MDS was published recently (Faderl 2008a). Although this may be a major breakthrough for clofarabine, I would not yet recommend it as front-line therapy for AML. We need to evaluate the data before we start replacing “7 + 3” (anthracycline-based therapy with cytarabine) with clofarabine.
I feel better now using a hypomethylating agent off protocol for a patient with AML in the front-line setting. In this setting, the mortality rates are lower and the toxicity profiles are better with hypomethylating agents.
![]() DR LOVE: What are the side effects and toxicities with clofarabine, either
alone or in combination with ara-C?
DR LOVE: What are the side effects and toxicities with clofarabine, either
alone or in combination with ara-C?
![]() DR GARCIA-MANERO: It is a powerful cytotoxic agent, so when we induce
patients with clofarabine we do so in the hospital in a protected environment,
not that different from the “7 + 3”-type approach, as opposed to induction
with a hypomethylating agent, which can be administered on an outpatient
basis. So that doesn’t change much.
DR GARCIA-MANERO: It is a powerful cytotoxic agent, so when we induce
patients with clofarabine we do so in the hospital in a protected environment,
not that different from the “7 + 3”-type approach, as opposed to induction
with a hypomethylating agent, which can be administered on an outpatient
basis. So that doesn’t change much.
Patients exhibit significant myelosuppression and neutropenic fever similar to those seen with an ara-C-containing regimen, in addition to the characteristic rash typically appearing on the patient’s upper body, which can be treated with steroids and topical care.
What changes is that the induction mortality drops from the 30 to 40 percent reported with a “7 + 3” regimen to approximately 10 percent with clofarabine (Atallah 2007; Faderl 2006), perhaps due to less mucosal damage that can lead to death from infection in patients receiving high-dose ara-C regimens. I believe that is what makes clofarabine attractive.
Tracks 6-8
![]() DR LOVE: Would you comment on the all-trans retinoic acid (ATRA)
and arsenic trioxide (ATO) combination therapy that’s used to treat acute
promyelocytic leukemia (APL)?
DR LOVE: Would you comment on the all-trans retinoic acid (ATRA)
and arsenic trioxide (ATO) combination therapy that’s used to treat acute
promyelocytic leukemia (APL)?
![]() DR GARCIA-MANERO: The ATRA/ATO combination was rapidly moved into
clinical trials of front-line therapy, and now it is becoming the standard treatment
for APL. Molecular responses are robust, and the disease-free survival
is approximately 90 percent in the median follow-up data we currently have
available. This is satisfactory, as this is a nonchemotherapy approach for APL.
DR GARCIA-MANERO: The ATRA/ATO combination was rapidly moved into
clinical trials of front-line therapy, and now it is becoming the standard treatment
for APL. Molecular responses are robust, and the disease-free survival
is approximately 90 percent in the median follow-up data we currently have
available. This is satisfactory, as this is a nonchemotherapy approach for APL.
The patients with higher-risk APL still pose a problem, largely because many of them die or develop a bleed in their brains before we can enroll them on studies. We do have a promising strategy of combining gemtuzumab ozogamicin with this ATRA/ATO regimen, and the outcomes are dramatic (Ravandi 2009).
The treatment protocols for the ATRA/ATO regimen with and without gemtuzumab ozogamicin are all available in the published literature. That being said, molecular monitoring is key in the treatment of these patients and you need access to a good RT-PCR assay for translocation 15;17 or the PML/ RARalpha fusions, otherwise you will be treating the patient blindly. Those tests are strong predictors of what will happen to the patient and should be repeated regularly during the course of therapy.
![]() DR LOVE: What do you see in terms of side effects and toxicity with this
therapy?
DR LOVE: What do you see in terms of side effects and toxicity with this
therapy?
![]() DR GARCIA-MANERO: The most important side effect of ATRA would be
the retinoic acid syndrome, which is basically a capillary-type syndrome with which, after a few days of therapy, patients develop pulmonary edema, shortness
of breath, et cetera. Nowadays this is rare, partly because the therapy
is changing the natural history of the disease and also because we heavily
premedicate patients with steroids. If the syndrome does occur, you can stop
therapy and treat them with the steroids, and most will fare well.
DR GARCIA-MANERO: The most important side effect of ATRA would be
the retinoic acid syndrome, which is basically a capillary-type syndrome with which, after a few days of therapy, patients develop pulmonary edema, shortness
of breath, et cetera. Nowadays this is rare, partly because the therapy
is changing the natural history of the disease and also because we heavily
premedicate patients with steroids. If the syndrome does occur, you can stop
therapy and treat them with the steroids, and most will fare well.
One peculiar toxicity exists with ATO, which is QT prolongation and torsades de pointes. This occurs infrequently and is more common in African-Americans than in Caucasians. Guidelines suggest that you can rechallenge patients with ATO, but I would not feel comfortable with that. The good news is that I have a number of such patients whom I have switched to idarubicin/anthracycline or gemtuzumab ozogamicin/ATRA, and they are now five to six years out and are faring extremely well.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Gail J Roboz, MD
- Select publications
Stephanie A Gregory, MD
- Select publications
Sundar Jagannath, MD
- Select publications
Guillermo Garcia-Manero, MD
- Select publications