

| Tracks 1-22 | ||||||||||||||||||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 3, 5
![]() DR LOVE: What’s new in the treatment of lymphoma?
DR LOVE: What’s new in the treatment of lymphoma?
![]() DR GREGORY: I’ve seen many new developments, although I’m uncertain
whether any of them will replace our standard treatment approaches.
Everyone is trying to improve upon rituximab, but how do you do that
with a monoclonal antibody? Perhaps we can improve the attachment of the
monoclonal antibody to the FC-gamma receptors from the monocyte-macrophage
system to enhance cell destruction. We can add cytokines, such as
GM-CSF or interleukin, to the monoclonal antibody or humanize it to
achieve more effective antibody-dependent cellular toxicity or to complement
cellular lysis.
DR GREGORY: I’ve seen many new developments, although I’m uncertain
whether any of them will replace our standard treatment approaches.
Everyone is trying to improve upon rituximab, but how do you do that
with a monoclonal antibody? Perhaps we can improve the attachment of the
monoclonal antibody to the FC-gamma receptors from the monocyte-macrophage
system to enhance cell destruction. We can add cytokines, such as
GM-CSF or interleukin, to the monoclonal antibody or humanize it to
achieve more effective antibody-dependent cellular toxicity or to complement
cellular lysis.
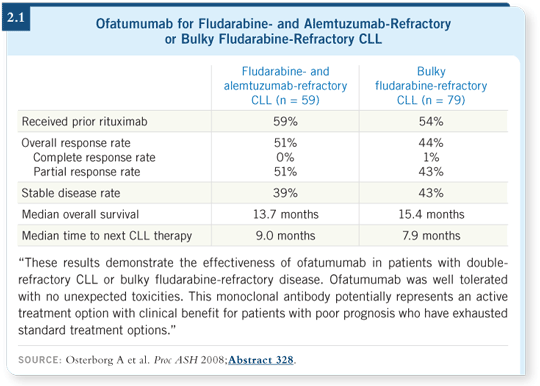
The novel agent ofatumumab is a humanized anti-CD20 monoclonal antibody currently in clinical trials for NHL and CLL. The data with ofatumumab mainly involve patients with relapsed/refractory CLL, and it appeared to be effective in some of the patients for whom a fludarabine/cyclophosphamide/rituximab (FCR)-type regimen had failed. It also appeared to work well for some of the patients with the poorer prognostic factors of CLL (Osterborg 2008; [2.1]).
In the front-line treatment of CLL, investigators will be conducting a trial comparing ofatumumab to chlorambucil, which is how bendamustine received FDA approval (Knauf 2008). They’re also evaluating ofatumumab in combination with fludarabine/cyclophosphamide in the relapsed setting.
Track 7
![]() DR LOVE: What are some recently reported data sets with bendamustine
in lymphoma?
DR LOVE: What are some recently reported data sets with bendamustine
in lymphoma?
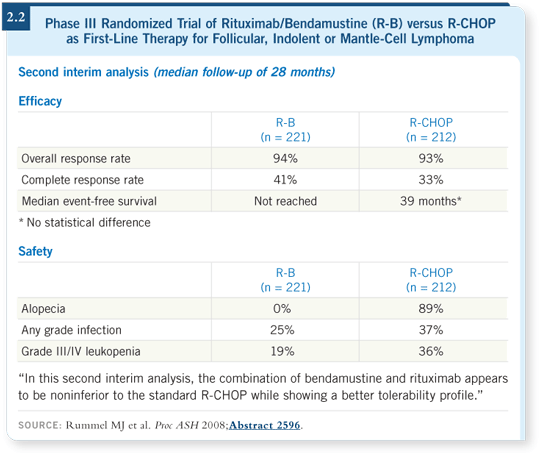
![]() DR GREGORY: One impressive study, reported by Dr Rummel, compared
bendamustine/rituximab to R-CHOP as front-line therapy for advanced follicular
or mantle-cell lymphoma. It was a noninferiority study, and he demonstrated
that bendamustine/rituximab appeared — at least in the interim analysis
— to be as effective as R-CHOP with less toxicity (Rummel 2008; [2.2]).
DR GREGORY: One impressive study, reported by Dr Rummel, compared
bendamustine/rituximab to R-CHOP as front-line therapy for advanced follicular
or mantle-cell lymphoma. It was a noninferiority study, and he demonstrated
that bendamustine/rituximab appeared — at least in the interim analysis
— to be as effective as R-CHOP with less toxicity (Rummel 2008; [2.2]).
If those results hold up, we may replace CHOP with bendamustine as frontline therapy. Many physicians in the United States are still administering R-CHOP as front-line therapy for follicular lymphoma. It would be nice if we had a less toxic replacement for that regimen, and bendamustine may work.
Brad Kahl’s study of 100 patients with rituximab-refractory, indolent NHL led to the approval of bendamustine in the relapsed setting. That trial reported impressive overall response rates and durations of response. Bendamustine works for patients with rituximab-refractory disease (Kahl 2007).
Track 9
![]() DR LOVE: What about the use of maintenance rituximab for patients with
indolent lymphomas? What studies are evaluating this issue?
DR LOVE: What about the use of maintenance rituximab for patients with
indolent lymphomas? What studies are evaluating this issue?
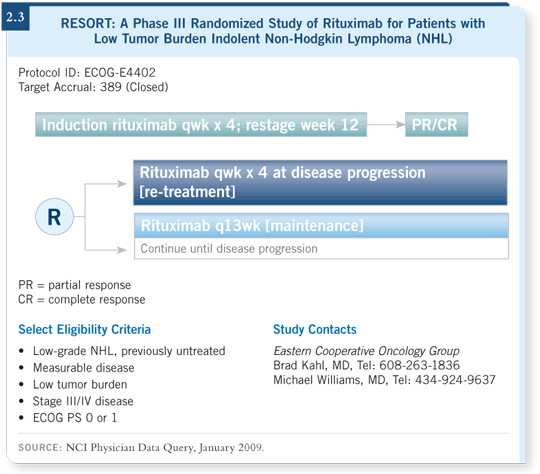
![]() DR GREGORY: The RESORT trial (ECOG-E4402) recently closed to accrual.
This study is evaluating four weeks of rituximab for patients with asymptomatic indolent lymphomas, including follicular lymphomas with a low tumor
burden. If the patient demonstrates a partial or complete response after four
weeks of rituximab, he or she is randomly assigned to one of two arms (2.3).
DR GREGORY: The RESORT trial (ECOG-E4402) recently closed to accrual.
This study is evaluating four weeks of rituximab for patients with asymptomatic indolent lymphomas, including follicular lymphomas with a low tumor
burden. If the patient demonstrates a partial or complete response after four
weeks of rituximab, he or she is randomly assigned to one of two arms (2.3).
One arm offers maintenance rituximab with one infusion every three months until the disease progresses. The other arm involves waiting until the patient experiences disease progression and then re-treating with four weeks of rituximab. If the disease progresses again, they re-treat again with four weeks of rituximab until the patient’s disease no longer responds.
At Rush University, we have enrolled five or six patients on this trial. We have four patients who are on the maintenance arm, and some of them have been receiving rituximab every three months and remain in complete remission for 4.5 to 5 years, which is impressive.
Is it better to keep patients on maintenance therapy or wait until the disease progresses and then re-treat? You’ll use much less rituximab with the second choice.
If it takes the same amount of time to become refractory to rituximab, what’s the sense in administering it every three months, spending money, suppressing the immune system and risking infection? As this question is currently being evaluated in the RESORT trial, I don’t have a conclusion.
Track 11
![]() DR LOVE: What other new agents are being evaluated for follicular
lymphoma?
DR LOVE: What other new agents are being evaluated for follicular
lymphoma?
![]() DR GREGORY: Lenalidomide is being evaluated, and bortezomib was recently
compared to bortezomib in combination with rituximab in relapsed/refractory
follicular lymphoma. An upcoming study will evaluate the combination of
rituximab, bendamustine and bortezomib. That should be an interesting trial.
DR GREGORY: Lenalidomide is being evaluated, and bortezomib was recently
compared to bortezomib in combination with rituximab in relapsed/refractory
follicular lymphoma. An upcoming study will evaluate the combination of
rituximab, bendamustine and bortezomib. That should be an interesting trial.
![]() DR LOVE: Would you discuss what we know about bortezomib in follicular
lymphoma?
DR LOVE: Would you discuss what we know about bortezomib in follicular
lymphoma?
![]() DR GREGORY: The trial with bortezomib/rituximab evaluated two different
ways of administering bortezomib to patients with relapsed/refractory disease.
One regimen was a weekly dose of bortezomib, and the other regimen was the
schedule used in multiple myeloma — on days one, four, eight and 11. Both
regimens seemed to yield good response rates, and it appeared that the weekly
infusion was less toxic (De Vos 2006).
DR GREGORY: The trial with bortezomib/rituximab evaluated two different
ways of administering bortezomib to patients with relapsed/refractory disease.
One regimen was a weekly dose of bortezomib, and the other regimen was the
schedule used in multiple myeloma — on days one, four, eight and 11. Both
regimens seemed to yield good response rates, and it appeared that the weekly
infusion was less toxic (De Vos 2006).
Track 16
![]() DR LOVE: How do you use bortezomib in the treatment of mantle-cell
lymphoma?
DR LOVE: How do you use bortezomib in the treatment of mantle-cell
lymphoma?
![]() DR GREGORY: I have used it a great deal in the relapsed setting on days one,
four, eight and 11. I often add rituximab on day one. I try to administer at
least six to eight cycles because I believe that if you give up after the first
couple of cycles, you haven’t completed an adequate trial period.
I have been relatively impressed with bortezomib in the relapsed setting. We’re
not talking about long responses. We’re talking about months, not years, but
it’s something to offer a patient who has experienced relapse. If the patient is
young, we try to find an allogeneic donor and perhaps perform a nonmyeloablative
allotransplant.
DR GREGORY: I have used it a great deal in the relapsed setting on days one,
four, eight and 11. I often add rituximab on day one. I try to administer at
least six to eight cycles because I believe that if you give up after the first
couple of cycles, you haven’t completed an adequate trial period.
I have been relatively impressed with bortezomib in the relapsed setting. We’re
not talking about long responses. We’re talking about months, not years, but
it’s something to offer a patient who has experienced relapse. If the patient is
young, we try to find an allogeneic donor and perhaps perform a nonmyeloablative
allotransplant.
Track 22
![]() DR LOVE: What else happened at ASH that’s important to know about?
DR LOVE: What else happened at ASH that’s important to know about?
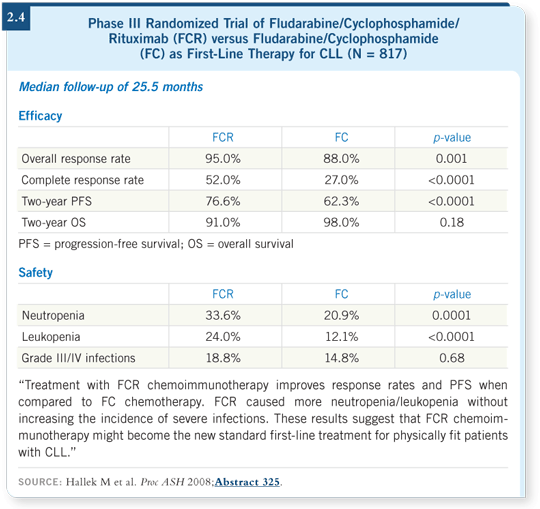
![]() DR GREGORY: The German CLL data were interesting. It was the first
randomized trial evaluating fludarabine/cyclophosphamide/rituximab (FCR)
versus fludarabine/cyclophosphamide (FC) as first-line therapy for CLL. The
results certainly favored FCR (Hallek 2008; [2.4]).
DR GREGORY: The German CLL data were interesting. It was the first
randomized trial evaluating fludarabine/cyclophosphamide/rituximab (FCR)
versus fludarabine/cyclophosphamide (FC) as first-line therapy for CLL. The
results certainly favored FCR (Hallek 2008; [2.4]).
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Gail J Roboz, MD
- Select publications
Stephanie A Gregory, MD
- Select publications
Sundar Jagannath, MD
- Select publications
Guillermo Garcia-Manero, MD
- Select publications