

| Tracks 1-14 | ||||||||||||||||||||||||||||||
|
Select Excerpts from the Interview
Tracks 6-8
![]() DR LOVE: Would you describe the design and key findings of the landmark study that demonstrated a survival advantage with azacitidine in MDS?
DR LOVE: Would you describe the design and key findings of the landmark study that demonstrated a survival advantage with azacitidine in MDS?
![]() DR ROBOZ: Results from the AZA-001 trial have now been presented at the past two major international meetings (Fenaux 2007; List 2008). In this study, patients with IPSS intermediate-2 and high-risk MDS were randomly assigned to treatment with azacitidine or a conventional care regimen (CCR).
DR ROBOZ: Results from the AZA-001 trial have now been presented at the past two major international meetings (Fenaux 2007; List 2008). In this study, patients with IPSS intermediate-2 and high-risk MDS were randomly assigned to treatment with azacitidine or a conventional care regimen (CCR).
The CCR was a choice of three approaches: supportive care, low-dose cytarabine or conventional induction/consolidation chemotherapy. Prior to randomization, the treating physician decided on the approach to CCR — whether the patient would receive induction chemotherapy, best supportive care with growth factors, antibiotics and transfusions or low-dose cytarabine. Once that decision was made, the patient was then randomly assigned to CCR or azacitidine.
All the important parameters, including survival, transformation to AML and major infections, favored the azacitidine arm (Fenaux 2007; Santini 2008; [1.1]). Evaluating outcomes according to the type of CCR is more difficult statistically.
Doctors and patients are asking, “How many cycles of this do I have to take? How long do I need this? If I’m not seeing a remission, according to the classic definition, is this drug still working?” These are important areas of ongoing research with azacitidine.
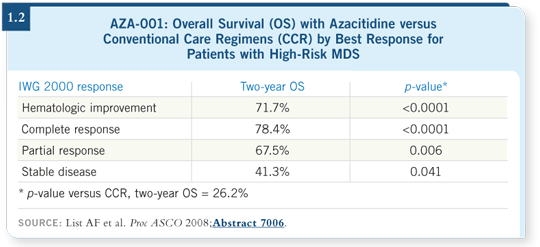
For the first time, we have convincing data that show that even if you are not meeting standard leukemia-type remission criteria — such as platelet counts higher than 100,000/μL, neutrophil counts higher than 1,000/mm3 — it’s possible that the survival benefit and the reduction in transformation to AML persist. As we obtain more data, it appears that we have good reason to maintain patients on azacitidine, even when not meeting conventional response criteria (List 2008; [1.2]).
When I initiate therapy, I don’t tell patients for how many cycles we will continue the treatment. I let them know that I would like them to receive at least four cycles of azacitidine, unless they are not tolerating it well, because I don’t believe that we can assess their response with fewer cycles. After four cycles, we’ll evaluate the counts, the patient’s general condition and how challenging the therapy has been. However, in general, I favor continuing the agent for now.
![]() DR LOVE: What about the magnitude of the benefit that was observed?
DR LOVE: What about the magnitude of the benefit that was observed?
![]() DR ROBOZ: Survival benefit is so difficult to determine in diseases for which a benefit measured in months rather than weeks already seems huge.
DR ROBOZ: Survival benefit is so difficult to determine in diseases for which a benefit measured in months rather than weeks already seems huge.
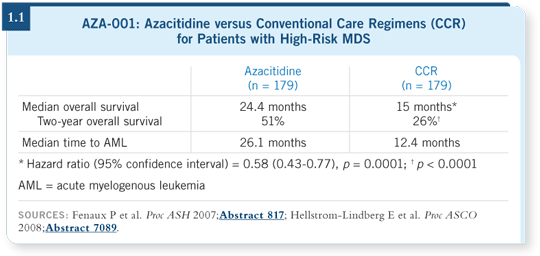
For patients with intermediate-2 and high-risk disease, responses that improve survival by close to nine months are significant (Fenaux 2007; List 2008). Oncology agents have been approved in other types of cancer for improvements measured in weeks, so magnitude-wise, this is significant.
It is an impressive number. However, in addition, because azacitidine is associated with an improvement in quality of life, patients are not simply surviving for a few more weeks but are rather experiencing meaningful improvements.
![]() DR LOVE: Does a certain subgroup of patients seem to have prolonged
responses, or are all patients only experiencing a small benefit?
DR LOVE: Does a certain subgroup of patients seem to have prolonged
responses, or are all patients only experiencing a small benefit?
![]() DR ROBOZ: We don’t know. In the past the patients at higher risk had more
profound responses, but the duration of benefit was not as prolonged.
DR ROBOZ: We don’t know. In the past the patients at higher risk had more
profound responses, but the duration of benefit was not as prolonged.
The initial studies with decitabine — another approved hypomethylating agent — included more patients with higher-risk disease, and the responses were good (Wijermans 2000; Kantarjian 2007a, 2007b). Some will argue that it is easier to induce a response in a patient with higher-risk disease due to increased cell turnover. It is easier to achieve remission in a patient with leukemia than one with MDS.
The questions that still come up are, What is the depth of the responses? Are the responses more durable in certain subgroups? We don’t know. Although a clear survival benefit has not been demonstrated with decitabine, it is structurally similar to azacitidine, and I’ve heard much discussion about whether the survival benefit is a class effect of hypomethylating agents or is specific to azacitidine.
![]() DR LOVE: How are you using decitabine in your practice?
DR LOVE: How are you using decitabine in your practice?
![]() DR ROBOZ: Although both drugs are approved for the same indications,
patients are aware that azacitidine has demonstrated a survival advantage
whereas decitabine has not. One could conjecture that if the clinical trials had
been conducted in exactly the same manner, then the results would be more
similar. Still, off study it’s probably easier to administer azacitidine to the
patients with intermediate-2 and high-risk disease, based on the new data.
DR ROBOZ: Although both drugs are approved for the same indications,
patients are aware that azacitidine has demonstrated a survival advantage
whereas decitabine has not. One could conjecture that if the clinical trials had
been conducted in exactly the same manner, then the results would be more
similar. Still, off study it’s probably easier to administer azacitidine to the
patients with intermediate-2 and high-risk disease, based on the new data.
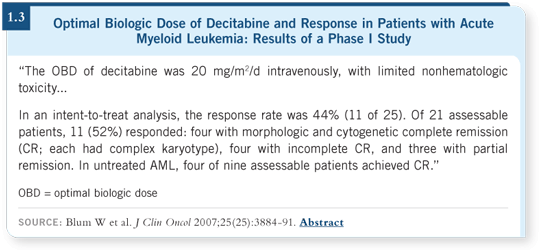
Decitabine is effective and well tolerated, and I’ve used it in an off-label manner for older patients with AML, based on some of the preliminary published data demonstrating responses when it’s used continuously for 10 days (Blum 2007; [1.3]).
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Gail J Roboz, MD
- Select publications
Stephanie A Gregory, MD
- Select publications
Sundar Jagannath, MD
- Select publications
Guillermo Garcia-Manero, MD
- Select publications