

| Tracks 1-9 | ||||||||||||||||||||
|
Select Excerpts from the Interview
Track 1
![]() DR LOVE: Can you discuss some of the important Phase III data sets presented at the 2008 ASH meeting?
DR LOVE: Can you discuss some of the important Phase III data sets presented at the 2008 ASH meeting?
![]() DR JAGANNATH: Paul Richardson from the Dana-Farber Cancer Institute
presented data on the RVD combination in patients with newly diagnosed
multiple myeloma (Richardson 2008).
DR JAGANNATH: Paul Richardson from the Dana-Farber Cancer Institute
presented data on the RVD combination in patients with newly diagnosed
multiple myeloma (Richardson 2008).
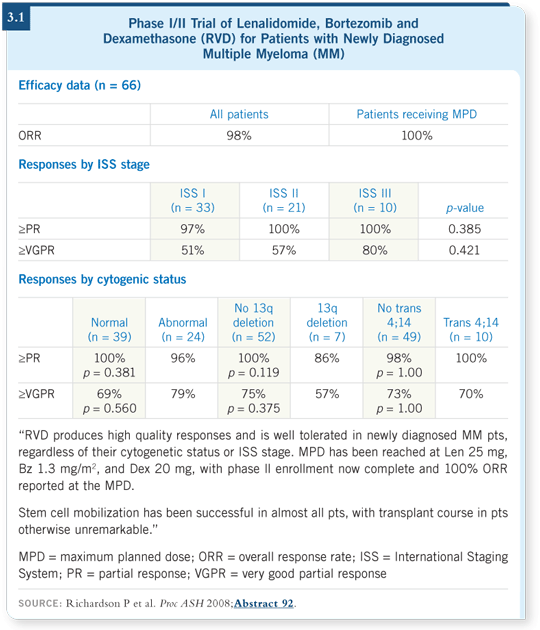
The previous year he presented data on the same regimen in patients with relapsed and refractory myeloma (Richardson 2007b), so the 2008 data were more of an update. The overall response rate for newly diagnosed patients who received the maximum planned dose was 100 percent (3.1).
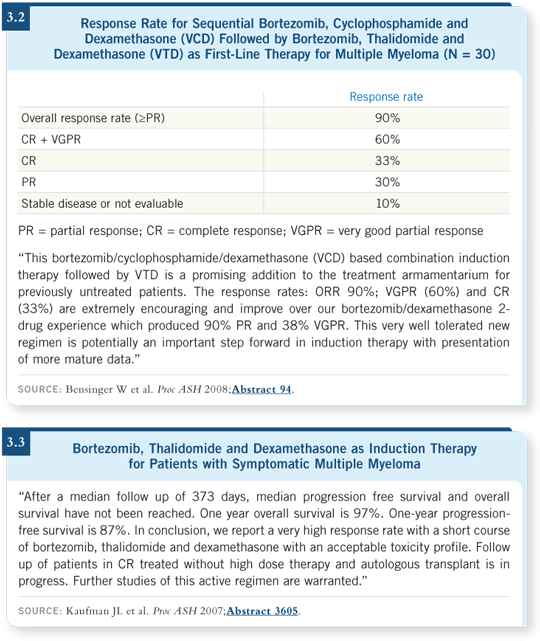
Similarly, Bill Bensinger presented data from a multi-institutional trial on the combination of bortezomib, cyclophosphamide and dexamethasone followed by bortezomib, thalidomide and dexamethasone as first-line therapy for multiple myeloma. In this trial, 90 percent of patients responded to the treatment (Bensinger 2008; [3.2]).
Another regimen consisting of bortezomib, thalidomide and dexamethasone as induction therapy for patients with symptomatic myeloma also demonstrated a good response rate (Kaufman 2007; [3.3]).
The data show that almost 100 percent of patients respond to these combinations, so for the first time we have agents in our armamentarium that have high response rates among newly diagnosed patients, and that is good news.
Track 2
![]() DR LOVE: What do we know about the effectiveness of bortezomib in
patients with high-risk disease?
DR LOVE: What do we know about the effectiveness of bortezomib in
patients with high-risk disease?
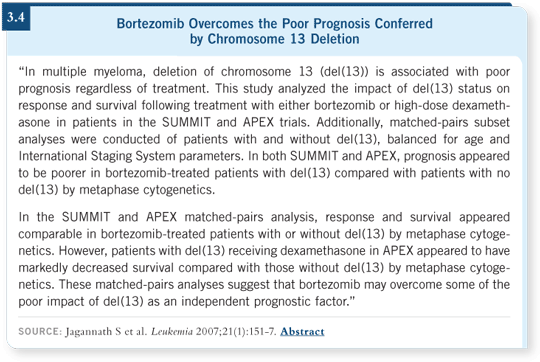
![]() DR JAGANNATH: The robust information that is being reported with
bortezomib shows that patients with multiple myeloma who are at high
risk — such as those who have 4;14 translocation, cytogenetically detected
chromosome 13 deletion, 14;16 translocation or 17p13 deletion — benefit from
the addition of this agent (Jagannath 2007; [3.4]). These patients have poor
outcomes when they are treated with traditional chemotherapy and simple autologous transplants, but whenever bortezomib is added to the mix, their outcomes dramatically improve.
DR JAGANNATH: The robust information that is being reported with
bortezomib shows that patients with multiple myeloma who are at high
risk — such as those who have 4;14 translocation, cytogenetically detected
chromosome 13 deletion, 14;16 translocation or 17p13 deletion — benefit from
the addition of this agent (Jagannath 2007; [3.4]). These patients have poor
outcomes when they are treated with traditional chemotherapy and simple autologous transplants, but whenever bortezomib is added to the mix, their outcomes dramatically improve.
We saw the benefit of this agent in the trial evaluating melphalan/prednisone with or without bortezomib as initial treatment for multiple myeloma (San Miguel 2008). The French Francophone Myeloma Intergroup (IFM) has also observed this in its trials of bortezomib/dexamethasone as induction therapy (Harousseau 2006, 2008).
As the addition of bortezomib apparently overcomes a poor prognosis, more and more physicians are using it for their patients who are at high risk. They are also using it for patients who have renal impairment or other comorbidities that increase their risk of developing deep venous thromboses, as that risk is minimal with bortezomib.
Physicians now have a choice of regimens when treating their patients with multiple myeloma. The Mayo Clinic website recommends that patients at high risk receive bortezomib and patients at low risk receive lenalidomide. However, I believe that will be questioned because we can now treat with the combination of bortezomib/lenalidomide/dexamethasone, and we don’t have to base our selection on whether the prognosis is good or poor.
Track 3
![]() DR LOVE: What have you observed in terms of the toxicity associated
with RVD?
DR LOVE: What have you observed in terms of the toxicity associated
with RVD?
![]() DR JAGANNATH: In the clinical trial evaluating RVD in patients with newly
diagnosed multiple myeloma, the regimen was well tolerated.
DR JAGANNATH: In the clinical trial evaluating RVD in patients with newly
diagnosed multiple myeloma, the regimen was well tolerated.
For some of the patients from whom we were able to collect stem cells, the effect of the lenalidomide persisted and cyclophosphamide was required for mobilization. So when lenalidomide is incorporated up front, collecting stem cells with filgrastim alone is not effective, but if you use two grams per meter squared of cyclophosphamide and the growth factor, it works well.
I believe that the RVD regimen is effective. Many of the patients on the trial have chosen not to undergo the stem cell transplant but rather to stay on the regimen and continue it at a lower dose as a maintenance phase.
This is an important clinical trial, and moving forward a study is being designed in which patients will receive four cycles of RVD and then either stay on the regimen or proceed to stem cell transplant followed by additional RVD, so all the patients will receive one year of this combination. We will be participating in this multi-institutional trial, along with the Dana-Farber and IFM groups, so I anticipate that patient accrual will be robust and quick.
| Table of Contents | Top of Page |
EDITOR
Neil Love, MD
INTERVIEWS
Gail J Roboz, MD
- Select publications
Stephanie A Gregory, MD
- Select publications
Sundar Jagannath, MD
- Select publications
Guillermo Garcia-Manero, MD
- Select publications